|
|||||
|
|
Introduction
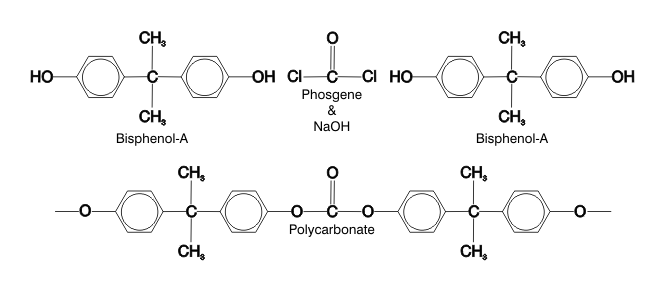
For many years, scientists have been warning that Bisphenol-A (see below) and many plasticizers (see below) are toxic. BPA is the primary ingredient of polycarbonate (PC)
Containers made of PET ![]() (polyethylene tere-phthalate, a polyester)
have been widely used and rigorously tested for use with foods and
beverages and found to be extremely safe.1 BetterBottle PET
plastics are not made with BPA or plasticizers. Nevertheless, articles claiming
that PET is unsafe and harmful to the environment keep popping up in the
media and on the Internet. Not infrequently, writers with insufficient
techhnical knowledge of chemistry or plastics have: a) Confused PET with
polyethylene, a completely different type of plastic; b) Concluded that
PET contains toxic ortho-phthalate plasticizers, because "phthalate"
appears in both names; or C) Lumped PET and polycarbonate together,
because both are tough, clear plastics. Sometimes, articles repeat utterly
foolish, long ago debunked, urban legends (see below). For example,
there's the legend about how PET containers cannot be washed and re-used -
nonsense. The following point-by-point discussion, citing references,
should help to dispell much of the confusion.
(polyethylene tere-phthalate, a polyester)
have been widely used and rigorously tested for use with foods and
beverages and found to be extremely safe.1 BetterBottle PET
plastics are not made with BPA or plasticizers. Nevertheless, articles claiming
that PET is unsafe and harmful to the environment keep popping up in the
media and on the Internet. Not infrequently, writers with insufficient
techhnical knowledge of chemistry or plastics have: a) Confused PET with
polyethylene, a completely different type of plastic; b) Concluded that
PET contains toxic ortho-phthalate plasticizers, because "phthalate"
appears in both names; or C) Lumped PET and polycarbonate together,
because both are tough, clear plastics. Sometimes, articles repeat utterly
foolish, long ago debunked, urban legends (see below). For example,
there's the legend about how PET containers cannot be washed and re-used -
nonsense. The following point-by-point discussion, citing references,
should help to dispell much of the confusion.
A word about winemaking and brewing ingenuity and
experimentation in general. Ingenuity and experimentation are an important
part of the home winemaking or brewing experience; however, unless a
person has specialized knowledge, it is not wise to use materials that
have not been approved for use with this class of beverages and the
related cleaning and sanitizing agents. For example, it was not very long ago that some winemakers were crushing
their grapes in old enamel bathtubs and basins. There is solid science
that this was, and is, a terrible idea. The glazes on old tubs and basins
are likely to contain toxic heavy metals that will dissolve in weakly
acidic grape juices.2
1 The Safety of Polyethylene Terephthalate (PET).
Plasticsinfo.org 2010, Plastics Division of the American Chemistry
Council, 1300 Wilson Blvd., Arlington VA 22209 (Accessed
05/15/10)
2 Mangas S, Visvanathan
R, van Alphen M. Lead Poisoning from Homemade Wine: A Case
Study. Enviro Health Perspec. 2001; 109:433-435 (Accessed
05/15/10)
So why to do suppliers of single-serve beverages, packaged in PET, tend to discourage reuse of their bottles? It is because they have concerns relating to sanitization, not the stability and safety PET. Single-serve bottles typically have small openings, so they are not washed effectively in a standard dishwasher. Unless automated bottle washing equipment is available, they must be washed and sanitized by hand. However, a wealth of practical experience indicates that many people are complacent and resort to a simple, quick rinse - often with nasty results.6 Drinking from a bottle, any type of bottle, will introduce microorganisms from your mouth and they will multiply in the remaining liquid or on the damp walls of the container. If a drink contains nutrients (e.g., flavor and nutrient enhanced water, juices, sports drinks etc.), microorganism growth will be much more rapid. PET bottles can be safely reused, provided: 1) They are carefully washed and sanitized between fillings; 2) They are handled in a sanitary fashion during re-filling; and 3) Re-filled bottles are treated the way "open bottles" containing a similar liquid should be handled (i.e., refrigerated and/or consumed in a reasonable period of time).2 In general, it makes good sense to pour from a bottle into a cup when drinking, unless the drink is to be consumed in a relatively short period of time.
Just to put a final lid on the issue of PET bottle reuse, it is worth noting that health organizations around the world actively recommend the re-use of PET bottles as part of the Solar Disinfection, SODIS, process. 7 Microbiologically unsafe water can be made potable by placing it in single-serve PET bottles and leaving the filled bottles in direct sunlight for a sufficient period of time. UV-A light penetrates the PET and water to kill microorganisms of all types. There is no evidence that toxic chemicals migrate (leach) from the PET. 2,8,9
1 Bottle Royale. Snopes.com (Accessed 05/15/10)
2 PET Safety FAQs National Association for PET Container Resources (NAPCOR) (Accessed 05/15/10)
3 Lilya D. Analysis and risk assessment of organic chemical migration from reused PET plastic bottles. (MScThesis Environmental Engineering). USA, University of Idaho, Environmental Science Program. 2001
4 Biscardi D, et al. Evaluation of the migration of mutagens/carcinogens from PET bottles into mineral water by Tradescantia/micronuclei test, Comet assay on leukocytes, and GC/MS. The Science of The Total Environment. 2003 (Jan 20); vol. 302 (Issues 1-3): pgs 101-108
5 Wegelin M, Schmid P. Migration of organic compounds from PET bottles. International Water and Sanitation Centre, Report Updated 07/23/03 (Accessed 05/15/10)
6 Oliphant JA, Ryan MC, Chu A. Bacterial water quality in the personal water bottles of elementary students. Canadian Journal of Public Health. 2002; vol. 93 (no. 5): pgs 366-367 (Accessed 05/15/10)
7 Wikipedia - Solar water disinfection (Accessed 05/15/10)
8 Berg M, Hug S. Water Treatment Technologies in Low Income Water Treatment Technologies in Low Income Regions Lecture: Water Resources and Drinking Water. EAWAG, Swiss Federal Institute for Environmental Science and Technology, Switzerland (Accessed 05/15/10)
9 Wegelin M, et al. Does sunlight change the material and content of polyethylene terephthalate (PET) bottles? Aqua. 2000; vol. 50: pgs. 125-135. (Accessed 05/15/10)
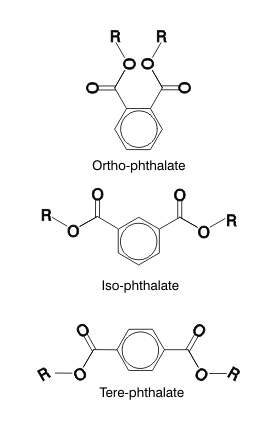
Phthalates
DEHP
DEHA
 Unlike many other types of plastics, virgin PET does not
contain plasticizers.1 - Plasticizers are added to make
plastics soft or flexible.2 For example, semi-rigid, polyvinyl
chloride (PVC or vinyl) plastic typically contains about 10 percent by
weight of plasticizers and very soft, polyvinyl chloride tubing may
contain as much as 80 percent. Plasticizers migrate out of plastics and
they are widely distributed in the environment.
Unlike many other types of plastics, virgin PET does not
contain plasticizers.1 - Plasticizers are added to make
plastics soft or flexible.2 For example, semi-rigid, polyvinyl
chloride (PVC or vinyl) plastic typically contains about 10 percent by
weight of plasticizers and very soft, polyvinyl chloride tubing may
contain as much as 80 percent. Plasticizers migrate out of plastics and
they are widely distributed in the environment. Phthalate plasticizers - There are three classes of phthalate esters, ortho-, iso-, and tere-, which differ by the relative positions of the two ester groups on the benzene ring. "ortho" refers to the fact that the side chains are adacent to one another on a central benzene ring. The side chains of "iso-" and "tere-" phthalates are further apart on their cental benzene ring. And in chemistry, small differences in structure can have a huge impact on how a chemical reacts. Ortho-phthalate plasticizers are metabolized quite rapidly to mono-esters (i.e., the replacement of a single "R" carbon chain with a hydrogen atom). These mono-esters are toxic and not readily metabolized further. Iso- and tere-phthalates are also metabolised quickly, but to iso- and tere-phthalic acid (i.e., both "R" carbon chains are replaced with hydrogen atoms), which is excreted and does not have the toxicity of the mono-ester metabolite of the ortho-phthalates.
Di(2-ethylhexyl) phthalate, DEHP, benzyl butyl
phthalate (BBzP), and dibutyl phthalate (DBP) are examples of very popular
ortho-phthalate plasticizers used in a wide variety of extremely poplular
plastic products (e.g., construction materials, food packaging, children
toys, medical devices, cling wrap, plastic tubing . . .). There is reason
to believe that ortho-phthalates have been associated an increased risk of
cancer, weakening of immune responses, disruption of sexual development,
reproductive system damage, and interfering with neurological
development.3,4,5,6,7 Industry groups argue that
ortho-phthalates are not a significant health risk; however, the US and
other countries are slowly beginning to limit the use of ortho-phthalates
and manufacturers of plasticized plastics are making major efforts to find
ways of preventing the plasticizers from oozing out of their
products.8 The US Consumer Product Safety Improvement Act of
2008, which is being appealed to various degrees, states that DEHP, BBzP,
and DBP may not be present in children's products and child care articles
at concentrations exceeding 0.1 percent.9,10 Additionally,
products that are expected to be placed in a child's mouth must not
contain more than 0.1 percent of the iso-phthalate plasticizers diisononyl
phthalate (DINP), diisodecyl phthalate (DIDP), or di-n-octyl phthalate
(DnOP).
Some people mistakenly believe
that PET contains phthalate plasticizers. The tere-phthalate in PET is not
a plasticizer additive that can migrate, it is tere-phthalic acid that is
chemically bonded (ester polymerized) with ethylene glycol to make
extremely long, stable, insoluble chains of polyethylene-tere-phthalate -
PET. Pure PET is rigid and opaque due to the formation of crystals. In
order to make clear, flexible PET bottles, a certain amount of copolymer
(most commonly iso-phthalic acid or similarly angled
1,4-Cyclohexanedimethanol) is used to replace a percentage of
tere-phthalic acid or ethylene glycol; thereby, forcing random bends in
the chemical chains of the PET and preventing the formation of extensive
crystalization. Just to be absolutely clear, iso-phthalic acid and
1,4-cyclohexanedimethanol are not additives, they are chemically bonded
into the PET chains as copolymers - As
already stated, virgin PET does not contain
plasticizers.
Adipate plasticizers -
Di(2-ethylhexyl) adipate, DEHA, is an example of a very popular
adipate plasticizer that is used in a wide variety of plastic
products.2 Food wrap films contain about 25% by weight of DEHA.
Adipate plasticizers are considerably less toxic than ortho-phthalates;
however, there is evidence in rats that DEHA can disrupt fetal
development.4 DEHA is not inherent in
PET plastic as raw material, by product, or decomposition
product.
1 PET
Safety FAQs National Association for PET Container Resources (NAPCOR)
(Accessed 05/15/10)
2 Wikipedia -
Plasticizers (Accessed 05/15/10)
3 Wikipedia - Phthalates (Accessed 05/15/10)
4 Wikipedia - Dibutyl phthalate (Accessed
05/15/10)
5 Preliminary Report -
On The Safety of Medical Devices Containing
DEHP-plasticized PVC or Other Plasticizers on Neonates and Other Groups
Possibly at Risk. European Commission - Scientific Committee on
Emerging and Newly-Identified Health Risks (SCENIHR) Plenary Session June
21-22, 2007 (Accessed 05/15/10)
6
Engel SM et al. Prenatal Phthalate Exposure is Associated with
Childhood Behavior and Executive Functioning. Environ Health Perspect.
2010 published on line ahead of print publication 01/28/10 (Accessed
05/15/10)
7 Andrade AJ et al. A
dose-response study following in utero and lactational exposure to
di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low
dose effects on rat brain aromatase activity. Toxicology 2006 Oct 29;
227(3): pp 185-92 (Accessed 05/15/10)
8
Navarro R et al. Phthalate Plasticizers Covalently Bound to PVC:
Plasticization with Suppressed Migration. Macromolecules 2010; 43 (5):
pp 2377-2381 (Accessed 05/15/10)
9
Consumer Product Safety Improvement Act of 2008
(Accessed 05/15/10)
10 Prohibition on the Sale of Certain Products
Containing Specified Phthalates, Section 108 of the Consumer Product
Safety Improvement Act (CPSIA). U.S. Consumer Product Safety Commission
11/14/08 (Accessed 05/15/10)
PC is made by polymerizing BPA in the presence of sodium hydroxide (a strong caustic) and phosgene. All manner of food and beverage containers are made from PC, many of them intended for reuse; however, caustic degergents and even hot water can reverse the polymerization of PC to release BPA.5 This is reason for serious concern, because these are the very conditions used to wash and sanitize PC products. Many widely used dish washing detergents and sanitizers, such as sodium hypochlorite (bleach), are caustic.
By and large, recent literature on low-dose
effects of BPA split between industry-funded studies that find no
significant reasons for concern and publicly funded studies that find
plenty of reasons for alarm. 7,8 There is a wealth of
research demonstrating adverse genetic and endocrine effects at extremely
low concentrations - effects that may last for many
generations.2,8,9,10,11 There is also evidence that exposure to
BPA is related to an increased risk of cardiovascular disease, diabetes,
liver disease, and asthma.12,13,14
In April of 2008, Health Canada and the United
States National Institutes of Health (NIH) issued reports describing BPA
as dangerous.15,16,17 The NIH
concluded that for the most part people in developed countries have
measurable blood, tissue, and urine levels of BPA, levels which exceed the
low levels known to cause biological changes in animals. For all of these reasons, businesses are moving to discontinue
the use and sales of PC bottles and US and Canadian governmental agencies
are considering restrictions on the use of BPA.18-21
On October
18, 2008, the Canadian Government listed BPA as a toxic substance and
announced it would immediately proceed with drafting regulations to
prohibit the importation, sale and advertising of bottles containing
bisphenol-A and limit the amount released into the
environment.22 On October 31, 2008, FDA Commissioner Andrew von Eschenbach
announced that an FDA-commisioned panel of scientific experts was sharply
critical of an earler FDA report claiming that BPA is safe at current
levels found in plastic food containers. The panel accused the FDA of
relying too heavily on studies funded by the chemical industry and failing
to support its conclusion with scientific evidence.23 In May of 2009,
manufacturers of cans for beverages and foods and some of their biggest
customers, including Coca-Cola, met to devise a strategy to block looming
government bans of BPA. The plan appeared to call for hiring a pregnant
model to tour the country extoling the virtues of BPA.24
In 2010, a
group of scientists urged regulatory agencies to place much greater
emphasis on the actual measurement of BPA levels rather than relying
heavily on essentially theoretical models of BPA metabolism and uptake,
which understate the potential risks. 25 On a positive note, the
US Environmental Protection Agency (EPA) has added BPA to its chemicals of
concern list, beginning a process of examining the evironmental impact of
BPA.26
However, pro-BPA lobbyists have not given up by any means and their
continued efforts to spin the evidence was recently described in the
September 2011 issue of The Atlantic magazine. 27
In March of 2010, The FDA denied the Natural Resources Defense Council (NRDC) petition to ban some uses of BPA, such as in plastic food packaging and coated metal cans, claiming there was insufficient evidence that BPA was a problem. This decision seems to fly in the face of overwhelming evidence to the contrary and has led to suggestions that the FDA should remove "responsible for protecting the public health" from its mission statement.28
In September of 2012, scientists reported a link between urinary BPA levels and obesity in young adults.29
In October of 2012, Jonathan Chevrier, PhD, of the University of California Berkeley School of Public Health, and colleagues reported that for every doubling in urinary BPA concentration, measured in women during the second half of pregnancy, there was an associated reduction in total thyroxine of 0.13 µg/dL30
1 Dianin AP. Zhurnal russkogo fiziko-khimicheskogo
obshchestva.1891; vol. 23: pg. 492.
2 Wikipedia - Bisphenol-A (Accessed 05/15/10)
3
Wikipedia - Diethylstilbestrol (Accessed
05/15/10)
4 Concern over canned foods. Consumer Reports
2009; December: pgs. 54-55 (Accessed 05/15/10)
5 Wikipedia -
Polycarbonate (Accessed
05/15/10)
6 Carwile JL et al. Polycarbonate Bottle Use
and Urinary Bisphenol A Concentrations. Environ Health Perspect 2009;
117:1368-1372 (Accessed 05/15/10)
7 vom Saal FS, Hughes
C. An extensive new literature concerning low-dose
effects of bisphenol A shows the need for a new risk assessment.
Environ Health Perspect. 2005; vol.113 (Issue 8): pgs. 926-33. PMID
16079060. (Accessed 05/15/10)
8 vom Saal FS, Hughes C.
Bisphenol A: vom Saal and Hughes Respond.
Environ Health Perspect. 2006; vol.114(Issue1): pgs.A16-A17 (Accessed
05/15/10)
9 Hunt P, et al. Bisphenol A Exposure Causes Meiotic Aneuploidy in
the Female Mouse, Current Biology. 2003; vol.13: pgs.546-553
(Accessed 05/15/10)
10 Colborn T, et al. Our Stolen Future: How We Are Threatening Our
Fertility, Intelligence and Survival-- A Scientific Detective Story. Plume
Press 1997, (ISBN 0452274141) (Accessed 05/15/10)
11
Richter CA, et al. Estradiol and Bisphenol A Stimulate Androgen
Receptor and Estrogen Receptor Gene Expression in Fetal Mouse Prostate
Mesenchyme Cells. Environ Health Perspect. 2007; vol.115(Issue 6):
pgs. 902-908 (Accessed 05/15/10)
12 Meltzer D et al. Association of Urinary Bisphenol-A Concentration
with Heart Disease: Evidence from the NHANES 2003/2006. PLoS One.
2010; vol. 5, issue 1 e8673 (Accessed 05/15/10)
13 Nakajima
Y et al. Dose response of maternal exposure to
bisphenol-A(BPA) on the development of experimental astham in mouse
pups. J Allergy Clin Immunol 2010: 125: AB127 (Accessed
05/15/10)
14 Lang, IA, et al. Association of Urinary Bisphenol A Concentration
With Medical Disorders and Laboratory Abnormalities in Adults. JAMA.
2008; vol 300(11): pgs1303-1310 (Accessed 05/15/10)
15 Mittelstaed M. Canada first to label
bisphenol A as officially dangerous. Globe and Mail 04/15/08 (Accessed
05/15/10)
16 Draft NTP Brief on Bisphenol A. 04/14/2008; The
Center for the Evaluation of Risks to Human Reproduction (CERHR), National
Toxicology Program, National Institutes of Health, U.S. Department of
Health and Human Services. (Accessed 05/15/10)
17 NTP-CERHR Expert Panel Report on the Reproductive
and Developmental Toxicity of Bisphenol A. The Center for the
Evaluation of Risks to Human Reproduction (CERHR), National Toxicology
Program, National Institutes of Health, U.S. Department of Health and
Human Services. (Accessed 05/15/10)
18 Recalls &
Safety Alerts: A new focus on plastic ingredient in bottles and cans.
Consumer Reports May 2008, pages 10-11
19 Black D,
Gillespie K. Call for ban on chemical in baby bottles,
Toronto Star, Nov 21, 2007 (Accessed
05/15/10)
20 Mittelstaed M. NationalMountain Equipment pulls water bottles off
shelves - Country's largest specialty outdoor-goods retailer cites
concern over possible health risks. Globe and Mail 12/07/07
(Referrenced12/20/07)
21 Nurwisah, R. Lululemon pulls bottles containing bisphenol A off
shelves. National Post 12/18/07(Accessed 05/15/10)
22 Canada
adds bisphenol A to toxic list. The Star 10/18/2008 (Accessed
05/15/10)
23 Favole J. Review Reignites Questions Over BPA. The Wall
Street Journal 10/31/2008 (Accessed 05/15/10)
24 Layton L.
Strategy Being Devised To Protect Use of BPA.
The Washington Post 05/31/2009 (Accessed 05/15/10)
25
Vandenberg LN et al. Biomonitoring Studies Should Be Used by Regulatory
Agencies to Assess Human Exposure Levels and Safety of Bisphenol A.
Environ Health Perspect 2010 118:1051-1054. (Accessed
08/27/10)
26 Bisphenol-A Action Plan Summary. EPA July 27,
2010 (Accessed 08/27/10)
27 Grossman E. How Lobbyists Are Spinning Weak Science to defend
BPA. The Atlantic 2011; September 27 (Accessed
09/28/11)
28 Peebles, L. FDA "Wrong" Not to Ban BPA, Health Advocates
Say. Huffington Post 2012; March 30 (Accessed
04/20/12)
29 Leonardo Trasande, MD, MPP; Teresa M.
Attina, MD, PhD, MPH; Jan Blustein, MD, PhD. , Association Between Urinarty Bispehnol A
Concentration and Obesity Prevelence in Children and
Adolescents. JAMA 2012;308(11) 1113-1121 (Accessed
09/19/12)
30 Chevrie ,J et al.
Maternal Urinary
Bisphenol A during Pregnancy and Maternal and Neonatal Thyroid Function in
the CHAMACOS Study.. Environmental Health
Perspectives October 4, 2012 (Accessed
10/05/12)
Antioxidants
Slip additives
Antimicrobials
. . .
1 National Association for PET Container Resources (NAPCOR) Frequently Asked Questions (Accessed 05/15/10)
2 Halden R. Researcher Dispels Myth of Dioxins and Plastic Water Bottles. Johns Hopkins Bloomberg School of Public Health, Public Health News Center, 615 N. Wolfe Street, Baltimore, MD 21205 (Accessed 05/15/10)
Antimony trioxide, one of the most insoluble forms
of antimony, is used only as a catalyst in the production of PET.
Catalysts are substances that act in minute concentrations to promote
chemical reactions by lowering energy barriers for a chemical reaction;
they do not become part of the product, except as trace contaminants. Dr.
Bill Shotyk, who had been studying antimony in polar snow and ice at
concentrations of a few parts per trillion, found traces of antimony in
single-serve, PET bottled water and concluded that it was not advisable to
use PET containers to store his samples. Dr. Shotyk examined 12 brands of
natural waters from Canada and three brands of deionized water and
determined that they contained 156+/- 86 parts per trillion (ppt) Sb and
162+/- 30 ppt Sb respectively.5 The news media, not always the
most reliable group of scientific journals, picked up on what Dr. Shotyk
reported and attached attention-grabbing headlines.
Subsequently,
Dr. Shotky and others reported that antimony concentrations in water that
was stored in PET containers gradually increased with time, leveling off
at about one part per billion ( 1 ppb) after approximately three years of
storage at room temperature.6,7 This information was also
misinterpreted by the media and Internet sites. Yes, the levels of
antimony increased significantly with extended time and increasing
temperature. However, they remained at less than a couple parts per
billion, even for small, single-serve bottles, which represent the worst
case situation (small volume of water to relatively large surface area of
PET).
It is important to give this
issue some scientific perspective:
- One part per trillion (ppt) is the equivalent of 1 second in about 32,000 years.
- A part per million (ppm) is 1000 times greater than a part per billion (ppb), which is 1000 times greater than a part per trillion (ppt).
- The World Health Organization (WHO) has established a safe drinking water level for anitimony of 20 ppb (20,000 ppt) and the European Food Safety Authority has established a food level of 40 ppb (40,000 ppt).8
- US, Canadian, and European standards accept 5-6 ppb (5000-6000 ppt) levels in drinking water.9
- It is so difficult to measure levels of Sb in foods and beverages at levels of less than 1 ppb that there is relatively little data; however, according to a January 2000 Environmental Protection Agency (EPA) report, the average concentration of Sb in meats, vegetables, and seafood is 0.2 to 1.1 ppb (200-1100 ppt).10
- On average, an adult living in North America
takes in approximately 7.44 ug (7,440,000 trillionths of a gram) of
antimony each day. About 38% of this intake would be expected to come
from the consumption of water, typically municipal
supplies.11 The contribution to
this average by virgin PET bottles, and especially large carboys that
are washed and re-used, is negligible.
2 Veesnstra GE, Deyo J, and Penman M. The Oral Toxicity and Mutagenicity of Antimony Trioxide. Toxicology Letters 1998 vol. 95, sup. 1; page 136.
3 Oorts K and Smolders E. Ecological Threshold Concentrations for Antimony in Water and Soil. Environ. Chem. 2009 vol. 6(2);116-121. (Accessed 05/15/10)
4 Maher WA. Antimony in the environment - new global puzzle. Environ. Chem. 2009 vol. 6; pgs. 93-94. (Accessed 05/15/10)
5 Shotyk W, Krachler M, Chen B. Contamination of Canadian and European bottled waters with antimony from PET containers. J Environ Monit. 2006; vol.8: pgs. 288-292 (Accessed 05/15/10)
6 Westerhoff P et al. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Research: 2008 Volume 42, Issue 3, Pages 551-556 (Accessed 05/15/10)
7 Keresztes et al. Leaching of antimony from polyethylene terephthalate (PET) bottles into mineral water. Science of The Total Environment: Volume 407, Issue 16, 1 Pages 4731-4735, August 2009
8 Antimony in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality 2003). Geneva, World Health Organization (WHO/SDE/WSH/03.04/74). (Accessed 05/15/10)
9 Ground Water & Drinking Water: Consumer Factsheet on: ANTIMONY. United States Environmental Protection Agency (EPA); November 28th, 2006 (Accessed 05/15/10)
10 Technology Transfer Network - Air Toxics Web Site: Antimony Compounds (7440-36-0). United States Environmental Protection Agency (EPA); November 6th, 2007 (Accessed 05/15/10)
11 Water Quality and Health - Antimony: Exposure. Health Canada. Date Modified: 2008-01-07 (Accessed 05/15/10)
PET and our Planet - Debunking Urban
Legends
1 Chunkath, S.R. Hazardous Hues: Plastic vs. Glass. The Hindu, July of 2001 (Accessed 05//15/10)
2 Plastic Containers - The Backstory. National Geographic: Green Guide (Accessed 05/15/10)
3 Gerngross. T.U. and Slater, S.C. How Green are Green Plastics? Scientific American Aug 2000. (Accessed 05/15/10)
4 Benefits of Recycling to the Glass Manufacturer (Special focus on UK). GlassTec Topic of the Month - April 2008 (Accessed 05/15/10)
5 Costello, P.J. Saving Florida's Bikinis. RecyclingBizz.Com, 09/12/07 (Accessed 05/15/10)

